Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Baisheng Medical Co., Ltd
We are professional supplier of electrosurgical generators,electrosurgical pencil,electrosurgical pad,bipolar forceps,skin stapler,medical connecting cable,ECG electrode,physical treatment electrode.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

OBS Disposable Electrosurgical Pad with Cable
( Grounding Pad/Dispersive Electrode/Neutral Pad )
CE ISO Certified
Description:
OBS Disposable Electrosurgical Pad, also named ESU Pad, Grounding Pad, Neutral Plate, Patient Plate, and Dispersive Electrode etc., is approved by CE and ISO.OBS not only offers basic Electrosurgical Pads of monopolar and bipolar (solid and split foil) in upright and horizontal design fit for adult.
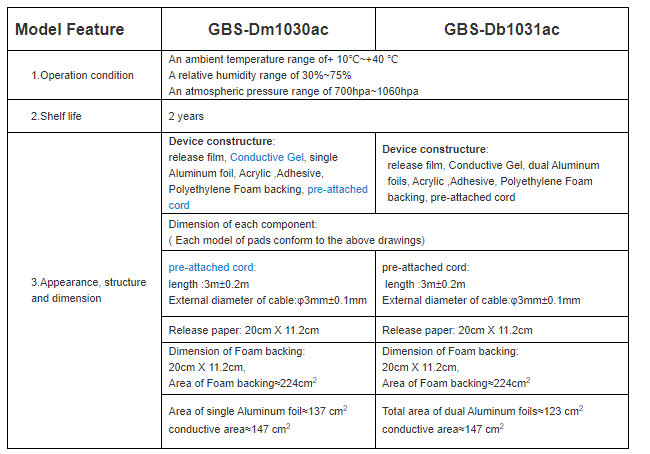
Product Features:
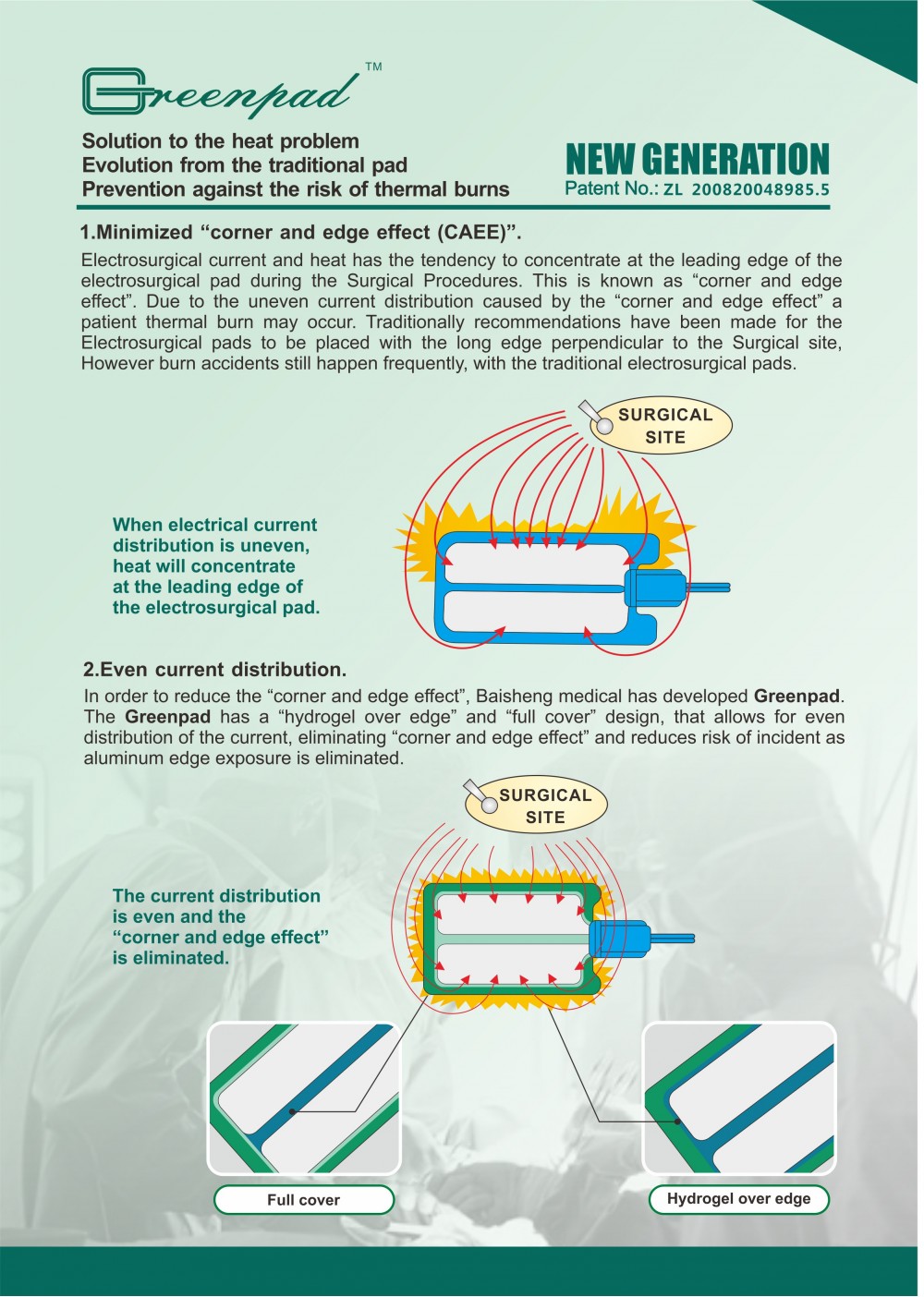
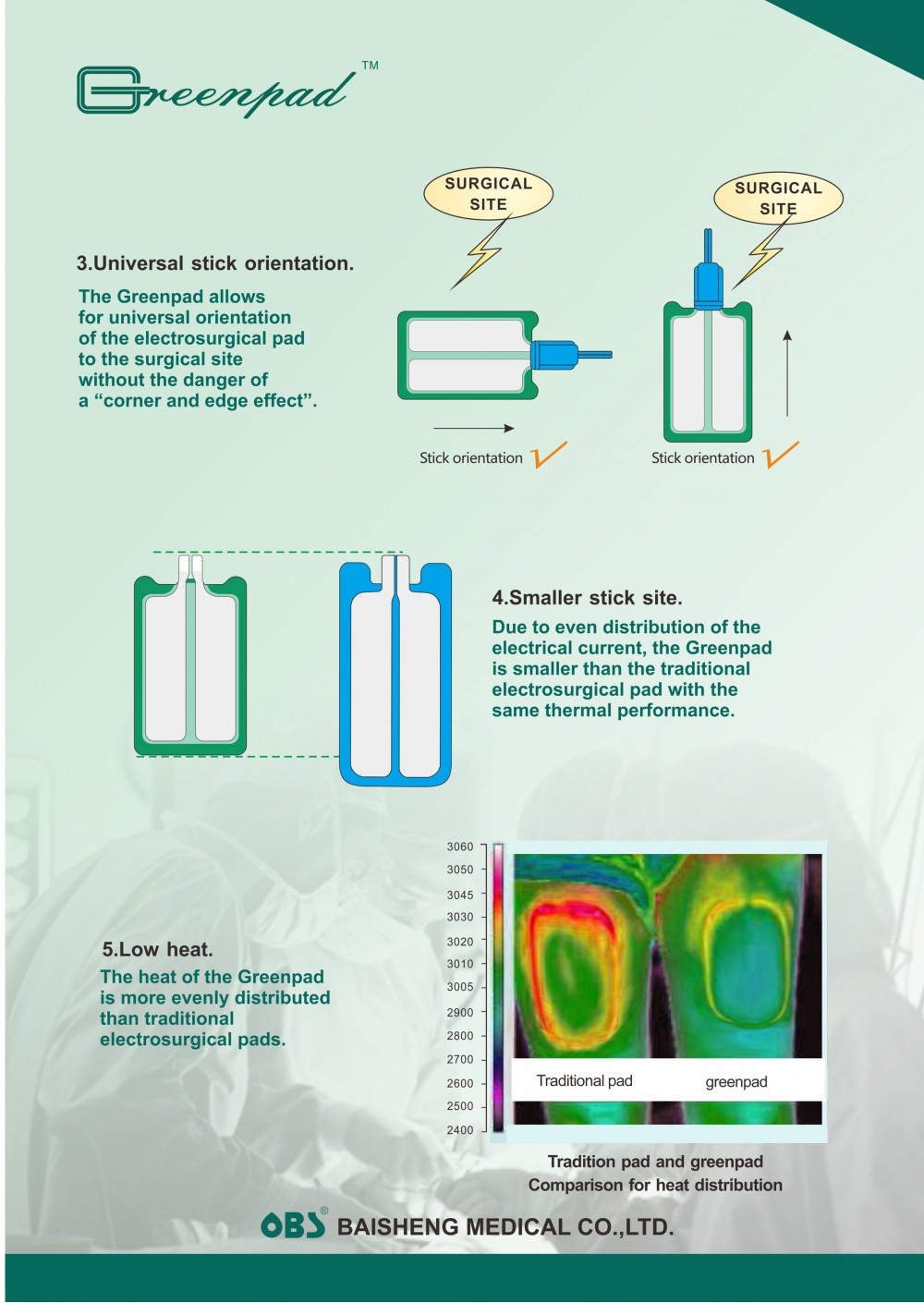
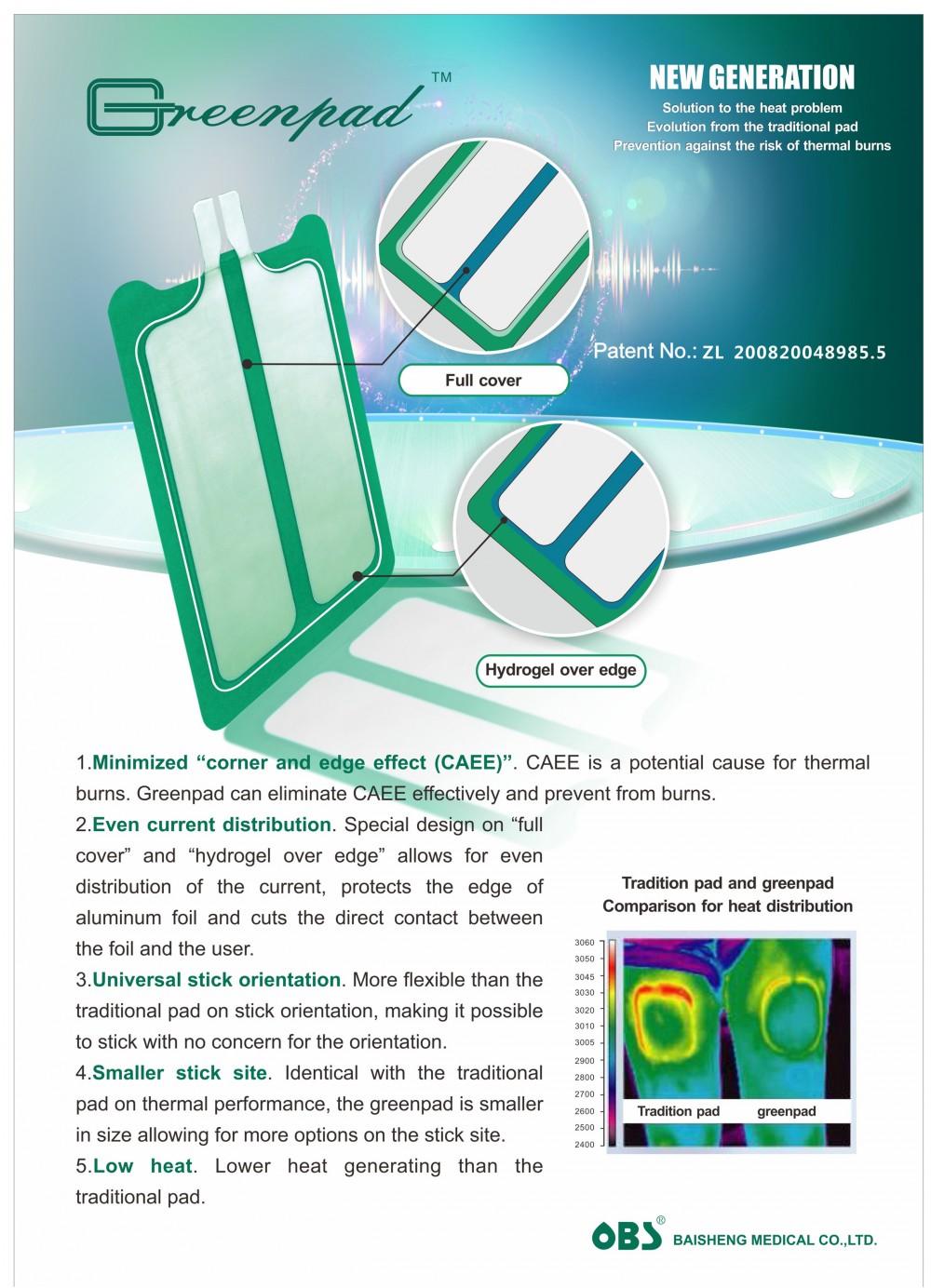

Packing:
1pc/pouch, 25 pouches/inner carton, 4 inner cartons/outer carton
Net weight: 9kg Gross weight: 10kg
Carton Size: 550*290*310mm

We are supplying electrosurgical pencil, electrosurgical pad, bipolar forceps and electrosurgical generator as well.






FAQ
Q: Are you a factory or a trading company?
A: We are a Chinese Medical instruments manufacturer. All the products are made of our workers.
Q: Can you accept the OEM?
A: Yes! We offer”OBS” brand to you. But if you want we can do the OEM service for you.
Q: Which areas are you products usually sold to?
A: Our products usually exported to North America, South America, The Middle East, South-east Asia, Europe and so on.
Q: What’s your deliver time?
A: If we have the stock, it will be delivered with 3 days. If it is very large, it will be delivered within 8-20 days.
Q:Why should you buy from us not from other suppliers?
Our company is focused on researching,designing,sailing which has above 25 years experience.Perfect after-sales service.CE, FDA certification.Highly cost-effective products, the same price and the best quality on the market!
Q. How can we guarantee quality?
We rely on the highest quality and production standards both when purchasing raw materials and during production.
Close ISO 13485 medical device quality management system from development through all production stages to the end product, with monitored cleaning and control procedures, cleanroom production and assembly, offers maximum process reliability.